
In addition to “dead Zn”, some dendrites could continually grow along with the separator until they pierce it and thus cause a short circuit. The inactive “dead Zn” with the insulating byproduct layer increases the internal resistance and polarization of the battery. In addition, the dendrites are prone to breaking away from Zn substrate and then becoming “dead Zn” due to the bad connection between the dendrites and anodes. Owing to the loose and porous 3D structure of Zn dendrites, more of the fresh Zn could contact with aqueous electrolytes, leading to more reaction sites for side reactions. The growing Zn dendrites would bring several hazards. These clusters can also serve as tiny protrusions with larger curvature and induce Zn dendrite growth due to the tip effect. The formed Zn atomic clusters disperse heterogeneously on the surface of Zn, which would further exacerbate the uneven field distribution in turn. Then, the Zn 2+ would nucleate on these sites to form Zn atomic clusters. As a result, Zn 2+ is more likely to adsorb and aggregate on the higher active sites under the unrestricted 2D Zn 2+ diffusion.
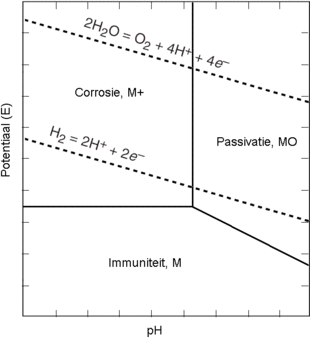
Specifically, the surface of Zn anodes is not atomically smooth, which could result in uneven electric field distribution, heterogeneous ion flux distribution, and different nucleation barrier sites on Zn anodes. These processes are susceptible to the surface microenvironment of Zn anodes. In the mild aqueous electrolyte, the reaction mechanism of a Zn anode can be summarized as follows:ĭuring electrodeposition, Zn 2+ typically undergoes four processes: adsorption, diffusion, nucleation, and growth. Finally, the challenges and further prospects of material designs for Zn anodes are put forward. The characteristics and functions of these materials on Zn anodes are discussed in detail. Subsequently, we focus on the material design strategies for the protection of Zn anodes and the high performance of AZIBs. In this review, we describe the origins and hazards of dendrite formation and side reactions of Zn anodes in mild AZIBs. However, a comprehensive review of strategies focusing on material designs for stabilizing the Zn anodes is still absent. These strategies effectively suppress the Zn dendrite growth and/or side reactions, thus being beneficial for enhancing the electrochemical performance of AZIBs. Therefore, some strategies have been developed to stabilize the Zn anodes, including surface modification, structural design, and electrolyte regulation, as presented in Figure 1. The two issues of Zn anodes exist simultaneously and promote each other, which seriously affects the reversibility of Zn chemistry and the electrochemical performance of AZIBs. The side reactions not only greatly increase the polarization but also reduce the Coulombic efficiency (CE) of the cell. In addition, the side reactions such as HER, corrosion, and passivation, driven by the contact between Zn anodes and aqueous electrolytes, are also regarded as big threats to AZIBs. The dendrites may pierce the separator and eventually cause the batteries to fail. Similar to the Li dendrites in lithium-ion batteries, the unlimited growth of Zn dendrites on Zn anodes is also a fatal hazard for AZIBs due to the nonuniform Zn nucleation and deposition. However, Zn anodes suffer from two main issues: dendrite growth and side reactions. Normally, Zn metal can directly serve as the anodes in AZIBs. The electrochemical performance of AZIBs mainly depends on the design of cathodes and anodes. These advantages make AZIBs have great prospects in the field of large-scale energy storage systems. Furthermore, Zn anodes with two-electron transfer characteristics possess high theoretical capacity (5855 mAh cm -3 and 820 mAh g -1), leading to high energy density. As a result, Zn metal can be directly used as the anode in aqueous electrolytes.

Besides, compared with alkaline metals and Ca/Mg/Al, Zn has suitable equilibrium electrode potential and high hydrogen evolution reaction (HER) overpotential in aqueous electrolytes. As a result, aqueous batteries often possess high power density. Moreover, higher ion conductivity can be obtained in aqueous electrolytes than the case in organic electrolytes. For instance, aqueous batteries are safe, cheap, and can be assembled in air. Mild AZIBs usually refer to the pH range of their electrolytes of ca. Among them, the typical cathode materials can be mainly divided into manganese-based oxides, vanadium-based oxides, Prussian blue analogs, and organic compounds. Common AZIBs are composed of Zn 2+ storage cathodes, Zn metal anodes, and aqueous electrolytes containing Zn 2+ salt. Rechargeable aqueous Zn-ion batteries (AZIBs) are regarded as a promising candidate for next-generation energy storage systems due to their remarkable advantages.


 0 kommentar(er)
0 kommentar(er)
